Greenfinch Technology is a Dublin based company, providing healthcare software development services for creating medical apps classified as Software as a Medical Device (SaMD) under the medical device regulations. Our team brings a wealth of experience in using the Microsoft technology stack and other technologies. We use the IEC 62304 software development process, helping to deliver software applications conforming to ISO 13485 regulations. Greenfinch Technology is an Irish software development company that specialises in SaMD projects. We work with medical companies in Ireland, the UK and as far away as America.
Daily Mood Diary
The Daily Mood Diary is an SaMD app for your phone. It is very useful when it comes to tracking your mood over an extended period. Used daily it can help to diagnose and address mental health issues. It addresses the challenge that many practitioners face when speaking with patients who are unable to recall accurately how they felt last week and who respond based upon their mood that day. This longitudinal approach ensures that the patient can accurately record fluctuations in their mental health and can share this with their mental health practitioner.
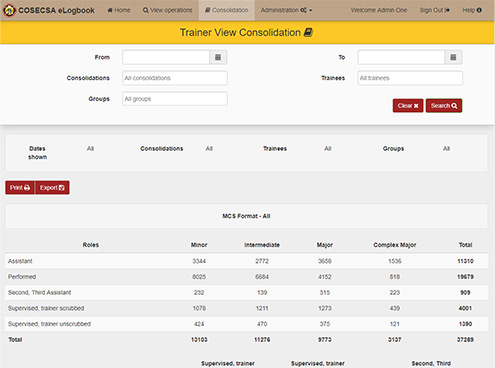
RCSI COSECSA
COSECSA is an initiative to help increase the number of surgeons in Africa. Greenfinch Technology has developed an electronic log-book SaMD app. This allows trainee surgeons to keep track of operations they were a part of either as the performing surgeon or as an assistant. It keeps track of all details to do with the surgery. Since 2007, RCSI and the College of Surgeons of East, Central and Southern Africa (COSECSA) have been working together to increase the number of trained surgeons and to improve the quality of surgical care in sub-Saharan Africa. This programme is funded by Irish Aid.
What is ISO 13485?
ISO 13485 is the International Organization Standardization for medical devices. This was first published in 1996 and it represents what is necessary for a complete quality management system when designing and manufacturing medical devices.
What is IEC 62034?
IEC 62034 is recognised in both the European Union and the USA as the international standard for developing medical device software. It is the benchmark that allows medical app development to comply with both these massive markets.
If interested in discussing having an SaMD developed we can be reached using our contact form.
Alternatively, call us on +353 (0)1 818 2949.
Recent projects
Recent projects
Daily Mood Diary
main features
RCSI COSECSA
main features
We deliver to ISO 13485 and IEC 62304 standards
Testimonials
Donncha Ryan, RCSI,
Stephen Dorman, MD,
Paul Boyle, CEO,
Partners

We are certified to help customers modernise their infrastructure, migrate applications and data to the cloud, and build cloud-based analytics solutions.

Greenfinch is the first Irish-owned company to be recognised as a Xamarin Premier Consulting Partner. Using one code base enables us to quickly and effectively deploy across multiple platforms.

As a Gold Competency Partner, we have access to the most up-to-date technology tools, training, and information to deliver resilient and stable apps.